Pharmaceutical and Analytical Study on Aranaladi Taila
DOI:
https://doi.org/10.47070/ijapr.v11i10.3005Keywords:
Sneha kalpana, Aranaladi taila, Pharmaceutico Analytical study, HPTLCAbstract
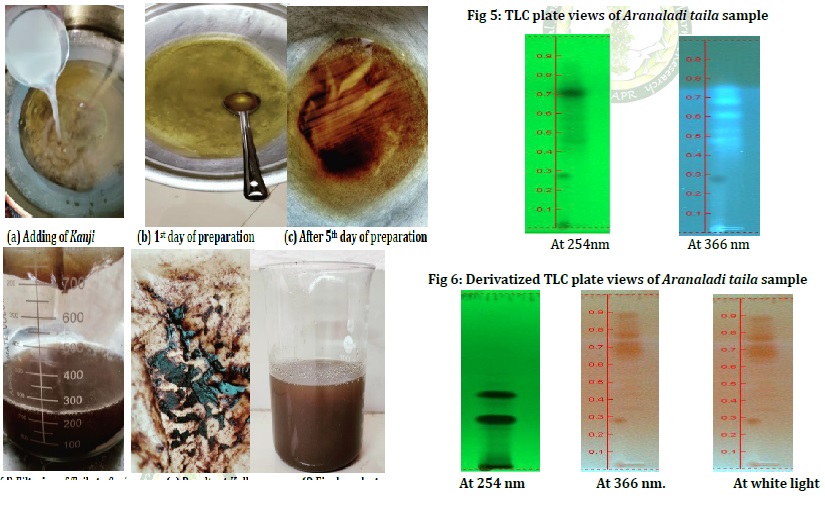
Rasashastra and Bhaishajya Kalpana is the branch of Ayurveda which deals with the preparation of herbal (Kashtoushadhis), mineral (Rasoushadhis) and herbomineral preparations. Bhaishajya Kalpana is a specialized branch which deals with the identification, collection, processing compounding and dispensing of Ayurvedic formulations. It comprises various dosage forms. Sneha Kalpana is a variant of Oushada Kalpana in which Sneha paka is performed using prescribed Kalka dravya and Drava dravya It includes Ghrita Kalpana and Taila Kalpana, among them Taila Kalpana is an important dosage form widely used in Ayurvedic industry. Aranaladi taila is an effective oil preparation mentioned in Sahasrayoga Taila prakarana, which contains Kanji, Tila taila and Sarjarasa. The term Aranala represents Kanji, which is the fermented form of rice water. By this process absorption of therapeutic active principles of the ingredients are ensured. In this formulation Kanji is the Dravadravya, Sarjarasa is the Kalka and Tilataila is the Sneha dravya. Aranaladi taila is indicated in Vatarakta associated with Jwara, Daha and Vedana. In the present study, an attempt was made to validate the pharmaceutical preparation of Aranaladi taila. The current study included a detailed pharmaceutical process and physico-chemical evaluation of Aranaladi taila. The physicochemical parameters included are Refractive index- 1.474, Acid value- 4.4, Iodine value- 97.4, Saponification value- 179.5 and Loss on drying- 0.40%w/w. HPTLC analysis revealed the variable number of spots, however, due to the absence of a standard marker compound the chemical constituent could not be identified.
Downloads