Pharmaceutico-Analytical and Antimicrobial Study of Amritank Rasa
DOI:
https://doi.org/10.47070/ijapr.v11i10.2954Keywords:
Amritank rasa, Kajjali, Trikatu, Pippalimoola, Chavya, Chitraka, Vatsanabha and Saindhava lavana, Kasa, Vati, Herbomineral formulation.Abstract
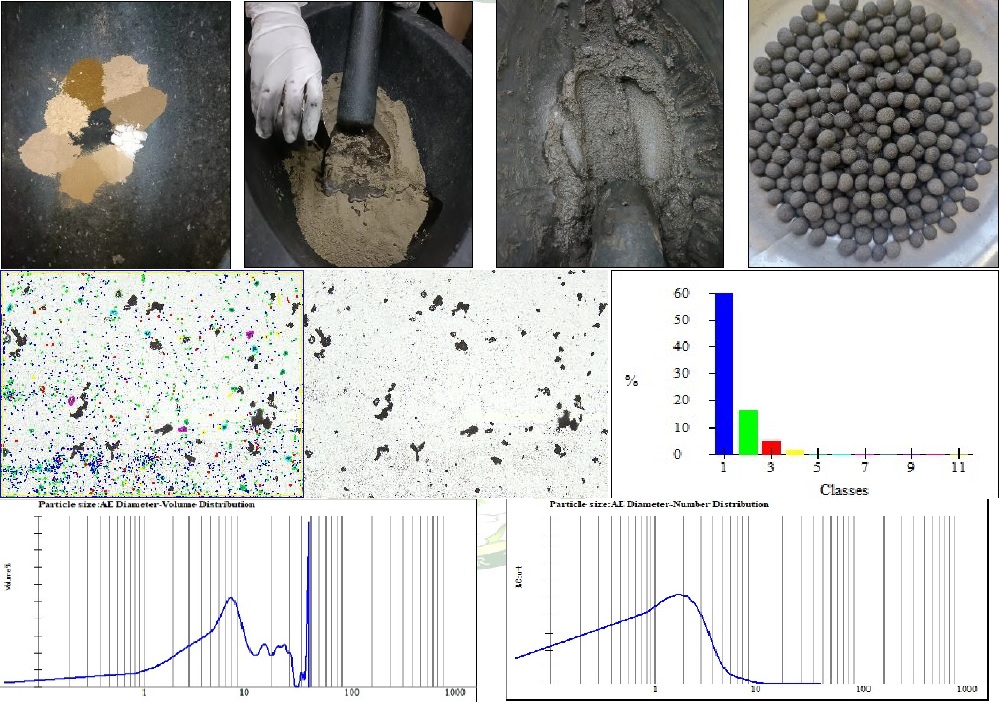
Amritank Rasa is a herbomineral formulation mentioned in the classical text of Basavrajiyam mainly used in the treatment of Pancha Kasa. This study aims to prepare the Amritank Rasa as per the classical text and conduct analytical and antimicrobial study of the prepared sample. The ingredients of Amritank Rasa are Kajjali, Trikatu, Pippalimoola, Chavya, Chitraka, Vatsanabha and Saindhava lavana all in equal parts and bhavana of Bhringaraj swarasa. The pharmaceutical procedure involves the preparation of Amritank Rasa vati in the 125mg dosage form. The pharmaceutical, analytical and antimicrobial parameters were compiled and data was recorded. The organoleptic parameters were, dark greyish in colour with pungent odour and taste, appearance was round and uncoated, and smooth in touch. The physiochemical parameters and quantitative parameters were, total ash was 5.5%, acid insoluble ash was 0.5% and loss on drying was 11.8%, average weight was 121mg, uniformity of weight test showed that all the tablets were not within the range of deviation from average weight, friability test was 0.51%, hardness was 5.3kg/cm2 and disintegration time test showed that any tablet did not dissolve in 60 minutes. The sample was then subjected to advanced analytical parameters i.e., FTIR, PSD, XRF and XRD. The antimicrobial study of Amritank rasa showed that it is effective against S.aureus, E.coli, P.aeruginosa and C. albicans. The development of the present study will serve as reference standards for Amritank Rasa formulation, quality control and clinical research
Downloads