An Overview on Vegetable Origin Drugs Used in Ayurveda, Included in the Schedule (E1) of the Drugs and Cosmetics Rules, 1945
DOI:
https://doi.org/10.47070/ijapr.v11i8.2869Keywords:
Ayurveda, The Drugs and Cosmetics Rules 1945, Visha-Upavisha varga (Poisonous drugs), Schedule (E1), Shodhana (Purification)Abstract
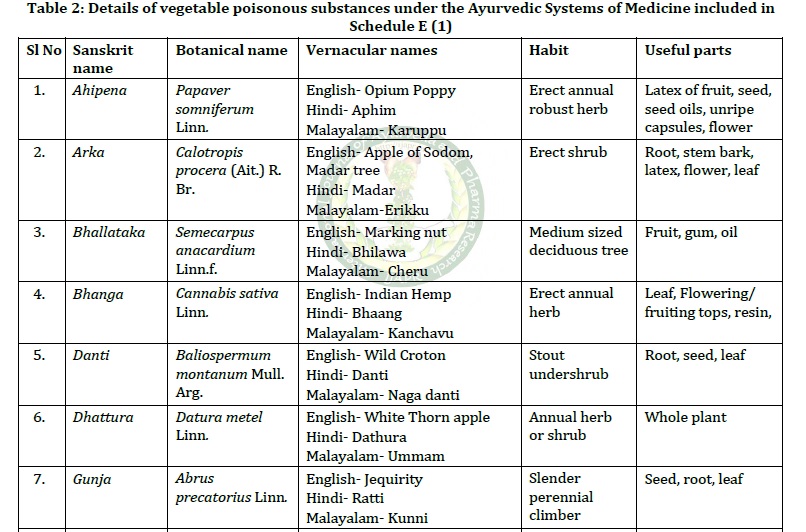
The Drugs and Cosmetics Rules, 1945 are the rules which Government of India framed under the Drugs and Cosmetics Act,1940. The objective of the act is to regulate the quality, safety and efficacy of the drugs and cosmetics sold in India. Schedule (E1) of the rules enlist the poisonous substances under the Ayurvedic (including Siddha) and Unani Systems of Medicine. The present work is an overview on the vegetable origin poisonous drugs used in Ayurvedic system of medicine. Methods: A thorough evaluation of literature was done, including the relevant portions of the Drugs and Cosmetics Rules, 1945, authoritative text books of Ayurveda, published research papers in reputed journals. Results: Schedule (E1) is related to Rule 161(2) of The Drugs and Cosmetics Rules, 1945; which instructs that if an ASU medicine contain any one of the Schedule (E1) drug as an ingredient, its label must contain a caution note, warning the user that it should be taken only under medical supervision. 14 vegetable origin drugs are categorized under the list of poisonous substances in Ayurvedic system. All these drugs have promising therapeutic utility which was also proved by various researches. Even though included in Visha-Upavisha varga (group of poisonous substances), these drugs are not toxic as Ayurveda advocates the unique processing method of Shodhana (purification) before using them therapeutically. Effect of Shodhana (purification) was also proved by various researches. Conclusion: Ayurvedic medicine, containing Schedule (E1) drug as an ingredient should be sold and used only under valid prescription of a registered physician. They are to be manufactured only after proper Shodhana (purification) of the poisonous ingredient. Caution label should be there on the medicine bottle. Physicians must ensure judicious usage of these medicines by giving proper patient education regarding the dosage and duration of administration.
Downloads