Evaluation of Nanocurcumin Mouthwash as an Adjunctive Plaque Control Agent: A Clinical Pilot Study
DOI:
https://doi.org/10.47070/ijapr.v10i4.2183Keywords:
Chlorhexidine, Local Drug Delivery, Mouthrinse, Nanocurcumin, PeriodontitisAbstract
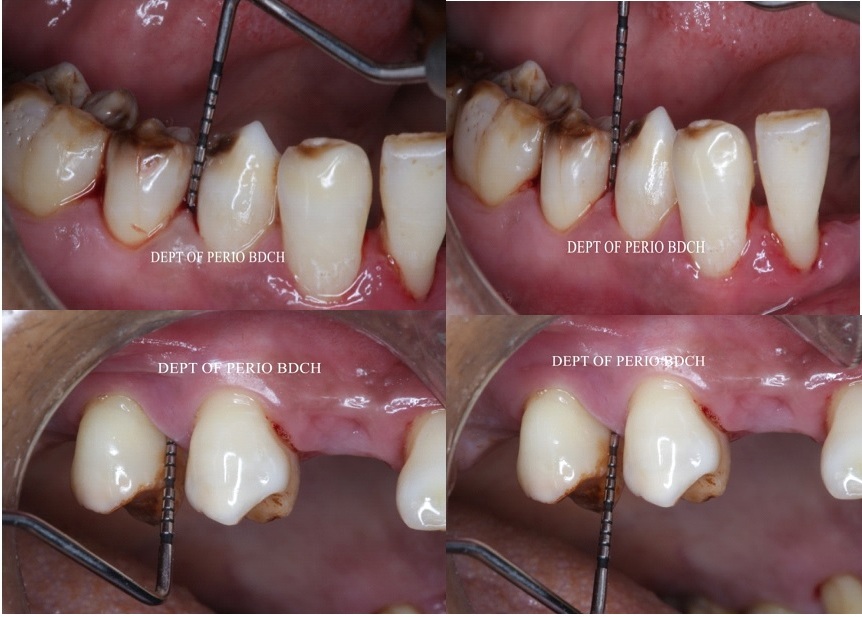
The use of adjunctive plaque control methods such as mouthwashes has shown to be effective in prevention of plaque accumulation. The gold standard for mouthwashes is 0.2% chlorhexidine, however, various side effects compels researchers to divert towards herbal alternatives. Curcumin has been enhanced with nanotechnology to enhance its efficacy and water solubility. The aim of study was to compare the efficacy of 0.1% nanocurcumin mouthwash to 0.2% chlorhexidine gluconate as an adjunct to scaling and root planning in localized chronic periodontitis patient. In this randomized controlled clinical study, a total of 30 patients having localized mild to moderate periodontitis were included. The study population was divided into 2 groups by randomization protocol; Group A (n=15) was given 0.1% nanocurcumin mouthwash whereas Group B (n=15) was given 0.2% CHX mouthwash. Clinical parameters including Plaque index (PI), Modified Gingival Index (mGI) being recorded at baseline, 30th day and 45th day and Sulcular Bleeding Index (SBI), Periodontal Probing Depths (PPD), Clinical attachment levels (CAL) were recorded at baseline and 45th day. Subjective criteria included taste acceptability, burning sensation and dryness whereas objective criteria including ulcer formation, tongue and teeth staining were analyzed. Statistically significant improvement was observed in all clinical parameters when compared to baseline in both groups and difference was statistically non-significant on intergroup comparison. No adverse reaction was observed in both groups in terms of subjective and objective criteria. Within the limitations, it can be concluded that nanocurcumin can be a viable alternative to chlorhexidine to formulate a mouthwash
Downloads